About Us
Under the management philosophy of 'A company that respects and love human beings',
we are sincerely striving to fulfill corporate social responsibilities with the goal of a young and healthy life.
- Supplied mainly prescription drugs in the hospital and clinic
- Exported about 60 items of products manufactured according to GMP standards of the Ministry of Food and Drug Safety to more than 13 countries around the world
- Awarded the 1 Million Dollar Tower on the 44th Trade Day in November 2007
- Consigned production of items certified as bioequivalence by the Ministry of Food and Drug Safety
-
0
Established year -
0
Number of medicines -
0
Number of export countries -
0
Number of export items
Headquarter

- Medica Korea Co., Ltd. declared its second start-up in 2000 with Dong-il New Pharmaceutical Co., Ltd., established in 1976, and sincerely strives to fulfill its corporate social responsibility with the goal of a young and healthy life under the management principle of a company with respects and love human beings.
We are exporting about 60 items to about 10 countries around the world with products manufactured according to GMP standards of the Ministry of Food and Drug Safety in accordance with stability and packaging techniques suitable for the climatic and pharmaceutical environment of the partner country.
We will become Medica Korea Co., Ltd. that develops one step further as a pioneer of the future pharmaceutical industry that accompanies healthy and beautiful lives of all citizens.
Hyangnam Factory
- Medica Korea Plant located in Hyangnam Pharmaceutical Complex located in 4th Grade 96, Hwasungnam-eup Pharmaceutical Complex, Hwasung City, Gyeonggi-do, KGMP plant with state-of-the-art production facilities on a 10,000m² site, produces over 340 medicines including specialty medicines and general medicines.
- In particular, the facility has been remodeled in December 2014 and has a specialized manufacturing facility capable of manufacturing solid medicines (tablets, capsules) in compliance with KGMP. All raw materials used in the Hyangnam factory are put into production through rigorous quality control process according to the KGMP regulations. Finished products are also shipped through strict quality control procedures.




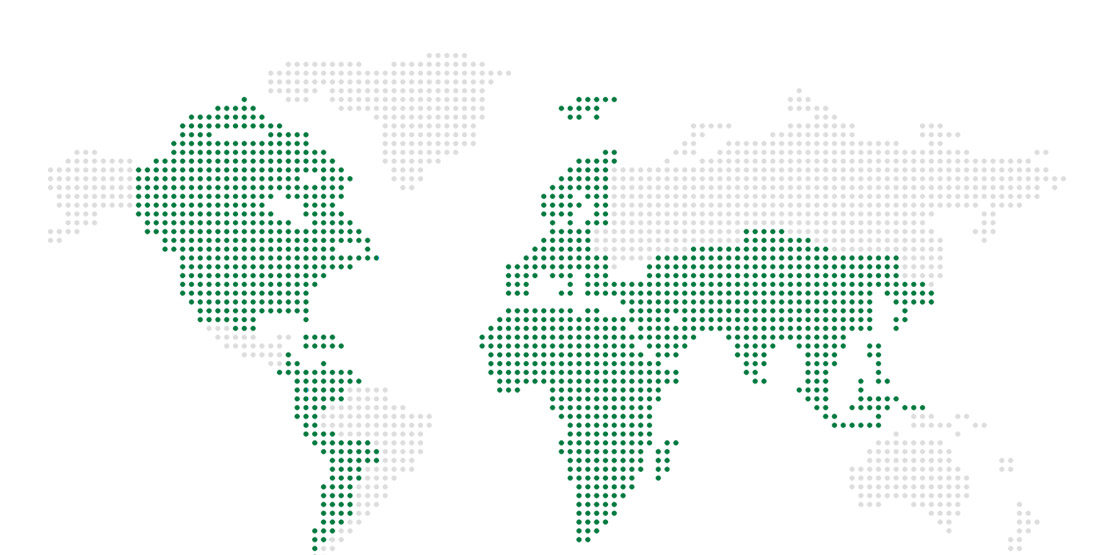
Export country
According to the Ministry of Food and Drug Safety's GMP standards, about 60 products are exported to 10 countries in the world and various products of excellent efficacy are provided in accordance with stability and packaging techniques suitable for climate and pharmaceutical environments. To increase exports, we are mainly dealing with products that have proven bioequivalence and are registering more than 50 new products in various countries. MK was selected as a 'Small and medium-sized enterprises with export prospects' in May 1995 and was awarded by an excellent export results on the 44th trading day in November 2007. We are making our way into the world with excellent products and personalized marketing and services from each country.
Certificate

Certificate of Youth-friendly Small Company

Certificate of World Class Plus Program Selection

Establishment of Industrial R&D Center

 KOR
KOR